Biologics- an Explosive trend in Pharma!
Biologics is the newest and most explosive trend in the pharmaceutical field, and here at Key Corporate Services, our Pharmaceuticals and Biotech Industry Executive Recruiter and Search teams are being kept busy placing elite professionals in biologic manufacturing and distribution.
As the need for top talent in the biologics marketplace today is growing, we interact at all stages of the process – with companies involved in drug discovery, development and commercial manufacturers of the compounds. Drawing upon our 15+ years experience in the pharmaceuticals market, we’ve been able to provide our Pharmaceuticals and Biotech Industry clients with job candidates who can make a real impact in their new positions.
Recruiting for the biological drug market focuses on positions in manufacturing and operations leadership for:
- branded drug manufacturers
- biosimilar manufacturers
- contract manufacturing organizations
- research firms
“What are biologics and how do they differ from more traditional drugs?
To answer that question, it’s important to first understand the way traditional drugs are made. Traditional pharma products are essentially chemicals synthesized from other chemicals. Each drug is made following a specific formula or “recipe”, combining specific chemical ingredients in an orderly fashion.
As Ed Zimney, MD of EverydayHealth.com explains, this process is usually done on an industrial scale. “Basically they take chemical A and add it to chemical B, mix it up, perhaps heat it a bit, add chemical C, perhaps filter it, etc., etc., until they get the final desire chemical in a pure form.”
Traditional pharmaceutical manufacturing, as any chemist can tell you, is nothing more than basic chemistry in action. Mix a little bit of this and a dash of that and voila, you end up with a drug! At any time, the finished drug can be broken down, analyzed, and its various components determined.
In fact, that “recipe” model accounts for the fact that competitors are able to create, and bring to market, competitive generics. Once the patent on the original traditional drug expires, generics manufacturers simply use these “copy-cat” recipes, which have the exact same chemical compounds as the original drug products. The manufacture of generics is of course done at much lower cost, since the manufacturers don’t have to recoup the huge expenses associated with drug discovery, development, clinical trials, and FDA approvals.
A biologic, by contrast, is no “copy”. Biologics are manufactured inside living systems, including:
- Microorganisms
- Plant cells
- Animal cells
Most biologics are very large, complex molecules or mixtures of molecules. Many biologics are produced using recombinant DNA technology, and therein lies the difference between biologics and traditional drug compounds.
“A variety of cells have been used to make biologics, but the key is that the cells have to be alive and fully functioning.” Biologics are made from “tricking” certain cells through biotechnology.”
Is biologics a totally new field?
Biologics are not new; development of human growth hormone, insulin, and red-blood cell stimulating agents occurred decades ago. First-generation biologics included things such as vaccines, blood and blood components. They were literally obtained from humans or animals, and used human blood, insulin from pigs or cows, or, in the case of the influenza vaccine, through growing the virus in chicken eggs.
It took a long time to bring these first-generation biologics to the market. Many early biotech companies failed. But, these failures were eventually followed by tremendous, market- changing successes. Companies such as Amgen ($AMGN), Genentech and Biogen Idec ($BIIB) were successful in bringing blockbuster products to the market. Their game- changing drugs turned those once-small players into giants.
These first generation products have given way to second-generation biologics, which rely on biotechnology for their manufacture. It’s this new generation of biologics that represent the future of medicine.
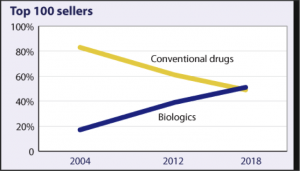
Conventional vs. biologic sales, worldwide
Percentage of sales attributable to each
Courtesy of managedcaremag.com
Conventional vs. biologic sales, worldwide
The last 20 years have seen a tremendous growth in biologic drug sales, as:
- The pharma industry moves into an era of personalized medicine
- pharmaceutical development and manufacturing activities are shifting their focus from traditional (small molecule) to biologic (large molecule) drug products.
Biologics have revolutionized the treatment of such chronic illnesses as:
- rheumatoid arthritis
- psoriasis
- psoriatic arthritis
- Crohn’s disease
- multiple sclerosis
- cancers
New product examples include:
- Enbrel
- Humira
- Remicade
- Avonex
- Betaseron
- Tysabri
- Cimzia
- Rituxan
- Neupogen
- Neulasta
- Leukine
Forecasts call for an explosive growth in the biological drugs market, and at Key Corporate Services, we are seeing requests for professionals in:
- bioanalytical laboratory testing
- active pharmaceutical intermediates
- mammalian cell culture manufacturing
- quality assurance
- regulatory affairs
- supply chain management
- business development
- project management
A recent report by CPhl Online, a pharma industry product and supplier site, presented a comprehensive overview of the phenomenal growth expected globally in biologic drugs through the year 2020. Experts in the industry are suggesting the biologic drug market will grow at an annual rate of 10.10% in the coming years.
Driving this growth are the changing demographics of our population. Globally, there is a growing geriatric population. In the U.S. alone, a large, growing segment of baby boomers are retiring, driving the need for new biologic drugs that can help them maintain active, healthy lifestyles for another 30-40 years. According to the report, the treatment and prevention of various diseases, such as blood related diseases; cancer, auto-immune diseases and other medical disorders are areas of focus for biologics. The advances in biomedical sciences are also fueling the potential growth of the biologic drugs market.
The numbers cited in the report highlight the skyrocketing growth anticipated in biologics over the next few years. While 2014 saw the biologic drug market in the US valued at $161 billion, it is estimated that the value of the same market will be $287 billion by 2020! That’s a growth of another $126 billion in just 6 years. This impressive growth is anticipated, in part, because biologics have already been very successful in treating those chronic diseases mentioned above. Funding support for research in biomedical science is coming from both public and private organizations. These market forces all combine to seek new ways to treat autoimmune diseases, cancer, diabetes, and cardiovascular diseases.
In Biologics, the product is the process!
Unlike the case with traditional drug processes, the finished product for a biologic cannot be fully characterized in the laboratory. Instead, biologic manufacturers must ensure product consistency, quality, and purity by ensuring that the manufacturing process remains substantially the same over time.
As experienced executive recruiters, we at KCS are seeing that difference in process translate itself into an increasing demand for expertise. While in traditional drug processes, changes require only an analysis of the finished product to establish that it is the same as before the manufacturing change, biologics require monitoring and innovative oversight at every step of the way.
“What ARE the processes?”
Making a biologic involves complex processes:
- The cells in which the product will be made have to be grown in extremely large quantities.
- Those cells must be maintained under conditions that allow them to live and function normally.
- From these large vats of cells, scientists must isolate the “right” gene needed to make the protein they want.
- Once this gene is isolated, it must be inserted into the host cell’s DNA, where it becomes permanent.
- Scientists then “plug in” some special bits that basically tell the cell that this gene is super-important and that the cell should churn out the desired protein. In essence, the cell has been “tricked” into becoming a specific protein-making mini-factory.
- Growing a large number of these dedicated protein machines results in a biologic product ready to be tested, then used by people who desperately need them.
It is a process that must be consistent and never changing.
Since biologics are dealing with living systems, which are sensitive to any changes to the manufacturing process, the normal flexibility afforded traditional drug manufacturing is not possible. Working with living organisms requires specialized processes that do not always resemble facilities, machinery, or equipment used to produce chemical drugs. And just small process differences can significantly affect the nature of the finished biologic and, most importantly, the way if functions in the body. Biologics manufacturers must tightly control the source and nature of starting materials, and consistently employ hundreds of process controls that assure predictable manufacturing outcomes.
The process control is specific to each biologic!
While such controls are basically the same for any drug company making the same “traditional” drug, that’s not the case in biologics. With biologics, process controls are established separately for and are unique to each biologic. Those controls are not applicable to another manufacturer.
Because these process controls may also be confidential to the origin manufacturer, it would be difficult or impossible for a second manufacturer to make the “same” biologic without intimate knowledge of and experience with the innovator’s process.
Production of biologics is expensive!
 Facilities for the production of biologics must be built from the ground up; you simply cannot adapt existing drug facilities to biologic production. With start up costs high, biologics are generally more expensive than traditional drugs.
Facilities for the production of biologics must be built from the ground up; you simply cannot adapt existing drug facilities to biologic production. With start up costs high, biologics are generally more expensive than traditional drugs.
Significantly, biologics manufacture demands a growing and high specialized and skilled labor pool.
The challenges to biologic production are daunting:
- The numbers of people to whom any one biologic can be marketed is comparatively small. The fewer the people to be treated, the harder it is to recoup the start-up costs. The high cost of pharmaceuticals, especially biologics, has become an important issue in the battle concerning ever-increasing healthcare costs. The average daily cost of a biologic in the United States is $45 compared with only $2 for chemical (small-molecule) drugs.
- The political and regulatory climate in which the drug companies must operate is a difficult one. Public acceptance for increasingly expensive medicine is tenuous, at best. More than one presidential candidate has voiced the voters outcry over rising, unbridled prescription drug costs. So, cost containment represents a real dilemma in biologics.
When thinking about the high costs associated with biologics, one of the first questions anyone might ask is “are generics available?” After all, about 80% of traditional drugs prescriptions written today are for generic versions.
So far, there are no generic versions of biologics available in the U.S, partly due to the expense of the manufacturing facilities, which will limit the number of companies able to get into this business. .
While generic biologic drugs are currently not available, biosimilars are. Biosimilars are less costly imitations of biologic drugs. While the FDA recognizes biosimilars as biologic’s version of a generic drug, they differ from generics in that they are not exact copies of the original biologics. Biosimilar manufacturers will generally need to generate data from lab testing, non-clinical testing and clinical testing to show that the biosimilar they have developed will provide the same therapeutic benefit and risks to patients as the referenced products. At KCS, we view these areas of biosimilar research, reporting, and marketing as generating increasing manpower demand.
While biosimilars have been available in Europe for a few years, September of 2015 saw the roll out of the first biosimilar approved by the FDA in the US. The drug, Zarzio, helps patients receiving chemotherapy make white blood cells, preventing infections common among cancer patients. It is considered therapeutically equivalent to the existing biologic, Neupogen.
The Sandoz.com website reports on the drug’s success: “Zarzio overtakes neurpogen and Granocyte to become the most prescribed Daily G-CSF in Europe.”
At Key Corporate Services, we recognize that not only is biologic production a complex process that includes finding the gene, inserting it into the cell, growing vast quantities of modified cells, and finally, siphoning off just the desired protein, it represents new employment opportunities that were nonexistent a decade ago.
A wide variety of highly trained scientists, marketing, distribution, and regulatory executives and employees is and will continue to be needed. That number far exceeds the numbers of people available from the ranks of existing pharmaceutical companies.
At KCS we’re convinced that biologics is ushering in a new era of pharmaceutical employment opportunity!